
AMONDYS 45 is used to treat patients with Duchenne muscular dystrophy (DMD) who have a confirmed mutation of the dystrophin gene that can be treated by skipping exon 45.
This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with AMONDYS 45. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
Embark on Beck’s journey with AMONDYS 45 as he and his family share their treatment goals and experiences, how AMONDYS 45 fits into their lives, and the vital role SareptAssist plays in their journey.
To receive AMONDYS 45, patients must have a mutation in the dystrophin gene that can be treated by skipping exon 45. Mutations are confirmed by a genetic test, which must be interpreted by a healthcare provider.
Individuals who are allergic to casimersen or any of the ingredients in AMONDYS 45 should not receive AMONDYS 45. Serious allergic reactions to casimersen have included anaphylaxis, which may include difficulty breathing, tightness in the chest, and angioedema which may include swelling of the mouth, face, lips, or tongue.
Serious allergic reactions, including angioedema (swelling under the skin, which may include mouth, face, lips, or tongue) and anaphylaxis (a serious, potentially life-threatening allergic reaction), have occurred in patients who were treated with AMONDYS 45. Patients should seek immediate medical care should they experience signs and symptoms of allergic reactions. Your doctor will institute appropriate medical treatment which may include slowing, interrupting, or discontinuing the AMONDYS 45 infusion. Your doctor will monitor you until the condition resolves.
Damage to the kidneys was seen in animals who received casimersen. Although damage to the kidneys was not seen in clinical studies with AMONDYS 45, potentially fatal kidney damage has occurred with other drugs that work in a similar way. Your doctor may recommend urine and blood testing before starting treatment followed by urine testing every month and a blood test every 3 months to monitor your kidneys.
Side effects occurring in at least 20% of patients treated with AMONDYS 45 and at least 5% more frequently than in patients who received an inactive intravenous (IV) infusion were (AMONDYS 45, placebo): upper respiratory tract infection (65%, 55%), cough (33%, 26%), fever (33%, 23%), headache (32%, 19%), joint pain (21%, 10%), and pain in mouth and throat (21%, 7%).
You should talk with your doctor about all the medications you are taking. Your doctor is the best person to advise you about your medications.
AMONDYS 45 is supplied in a 2 mL single-dose vial containing 100 mg casimersen (50 mg/mL). The solution is a clear to slightly opalescent, colorless liquid, and may contain trace amounts of white to off-white amorphous particles.
The amount of AMONDYS 45 you take is based on your weight. The recommended dosage of AMONDYS 45 is 30 milligrams of solution per kilogram of body weight, intravenously (IV) infused once a week over 35-60 minutes via an in-line 0.2 micron filter.
AMONDYS 45 is given by intravenous (IV) infusion once a week via an in-line 0.2 micron filter. An IV infusion is a way of delivering medicine directly into your bloodstream through a vein. Your doctor may discuss the use of a port, which is a device installed under the skin for repeat use in delivering IV medications. AMONDYS 45 infusion is always given and monitored by a healthcare provider.
AMONDYS 45 will be intravenously infused over 35-60 minutes via an in-line
You may receive your infusions at your doctor’s office, an infusion center, or your home. You and your doctor may need to discuss these options, including whether home therapy is an option for you.
If a dose of AMONDYS 45 is missed, it may be administered as soon as possible after the scheduled dose. Talk to your doctor if you miss a dose.
Ask your doctor for any patient instructions provided by the maker of your port. Carefully follow these or other instructions provided by your doctor for care of your port site to reduce the risk of complications including infections.
*Always refer to the port manufacturer's instruction for use (IFU) guide for more information on safety and precautions and ask your healthcare provider to review the relevant instruction for use of your port with you.
Proper hygiene is an important part of avoiding infection. You may be given instructions for keeping the port and surrounding skin clean after it is installed and after each time you use it.
*Always refer to the port manufacturer’s instruction for use (IFU) guide for more information on safety and precautions and ask your healthcare provider to review the relevant instruction for use of your port with you.
The instructions you get from your doctor will explain when you should contact them. Always contact your doctor if:
*Always refer to the port manufacturer’s instruction for use (IFU) guide for more information on safety and precautions and ask your healthcare provider to review the relevant instruction for use of your port with you.
Hypersensitivity Reactions: Serious allergic reactions, including angioedema and anaphylaxis, have occurred in patients who were treated with AMONDYS 45. Patients should seek immediate medical care should they experience signs and symptoms of allergic reactions. Your doctor will institute appropriate medical treatment which may include slowing, interrupting, or discontinuing the AMONDYS 45 infusion. Your doctor will monitor you until the condition resolves.
Kidney Toxicity and Monitoring: Damage to the kidneys was seen in animals who received casimersen. Although damage to the kidneys was not seen in clinical studies with AMONDYS 45, potentially fatal kidney damage has occurred with other drugs that work in a similar way. Your doctor may recommend urine and blood testing before starting treatment followed by urine testing every month and a blood test every 3 months to monitor your kidneys.
SIDE EFFECTS OBSERVED IN AT LEAST 20% OF PATIENTS TREATED WITH AMONDYS 45 AND AT LEAST 5% MORE FREQUENTLY THAN IN THE PLACEBO GROUP
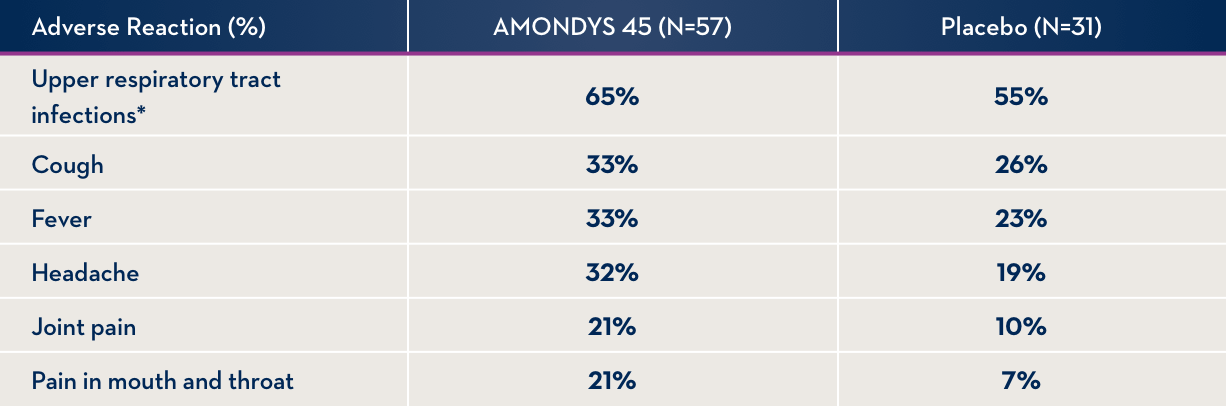
* Includes upper respiratory infection, infection of nose and/or throat, and stuffy or runny nose
Other side effects that occurred in at least 10% of patients treated with AMONDYS 45 and at least 5% more frequently than patients who received an inactive IV infusion were:
Infusion-related reactions including rash, headache, cough, abdominal pain (including upper abdominal pain), and vomiting occurred within 24 hours from the start of an infusion of AMONDYS 45.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Before receiving this infusion, please see the full Prescribing Information for AMONDYS 45 (casimersen).
A genetic test is required to confirm that a patient's mutation of the DMD gene is amenable to exon 45 skipping. For more information, explore the genetic testing resources at Duchenne.com.
AMONDYS 45 is an exon-skipping therapy. The goal of exon skipping is to allow the body to make a shorter form of the dystrophin protein.
Watch this video to learn how mutations in the dystrophin gene result in the body not being able to produce enough—or any—working dystrophin protein, and how exon-skipping aims to provide a solution by making a shorter form of the dystrophin protein.
The dystrophin gene is the largest gene in the body, made up of 79 exons (portions of a gene) that are linked together to form the instructions for making dystrophin — a protein muscles need to work properly.
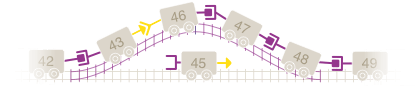
Think of the exons like toy train cars, each with a special connection that allows one car to connect to another. In order for all the cars to move together as a train, the connections between cars must match so that they can connect to one another.

Duchenne is caused by a genetic mutation, or change, in the dystrophin gene. Most commonly, one or more exons are missing. This causes errors in the instructions for making dystrophin, and the body is not able to produce enough or any working dystrophin protein.
Imagining the toy train, one or more cars would be missing, leaving the remaining cars not connected. In this example, we can see that car 44 is missing. This results in cars 43 and 45 not being able to connect.

Exon-skipping technology allows the body to make dystrophin protein by skipping over a specific exon. AMONDYS 45 works using exon skipping and the result is a shorter form of the dystrophin protein.
With our train, we would move a certain car aside to “skip over” it, so we could find a car with the right connection to allow the remaining cars to connect. In our example, car 45 would be skipped over to allow car 43 to connect to car 46. This new train would be shorter, but all the cars would still be connected.
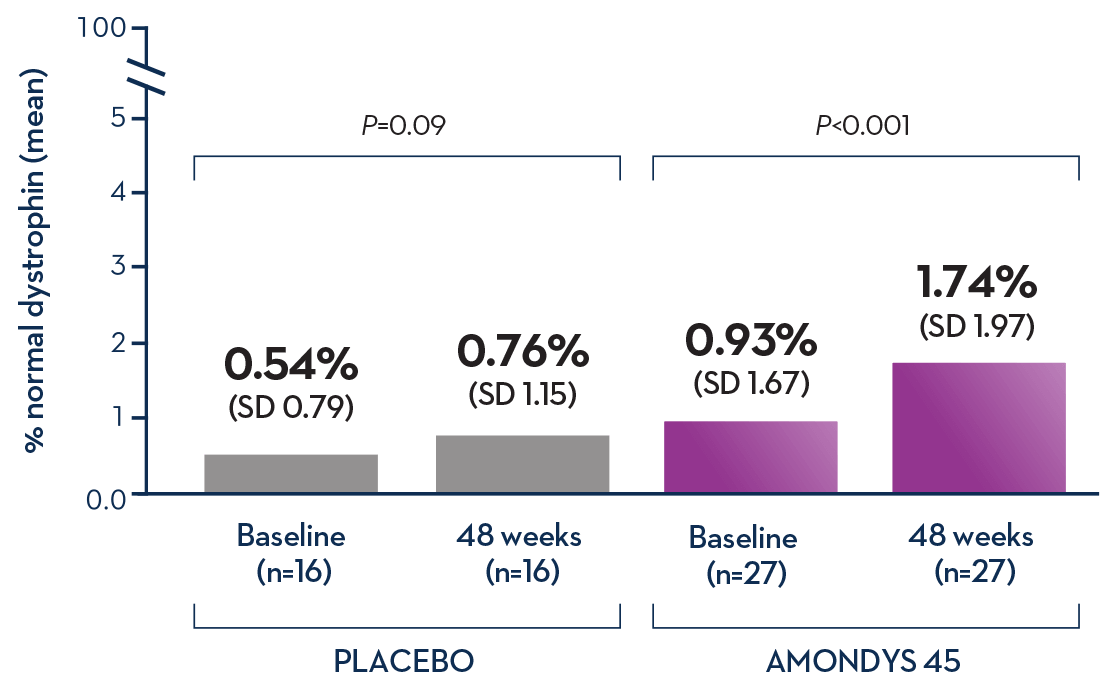
Boys who received AMONDYS 45 had variable responses in the amount of increased dystrophin production after 48 weeks of treatment. Data from an ongoing clinical study showed 27 boys (median age 9 years) who received AMONDYS 45 had an average increase of 1.74% of normal dystrophin production compared to 0.76% of normal production for boys who received a placebo infusion (n=16).
Join Beck and his family as they navigate living with Duchenne muscular dystrophy. Discover Beck's diverse interests, his contributions to the Duchenne community, and the motivation behind his continued treatment with AMONDYS 45.
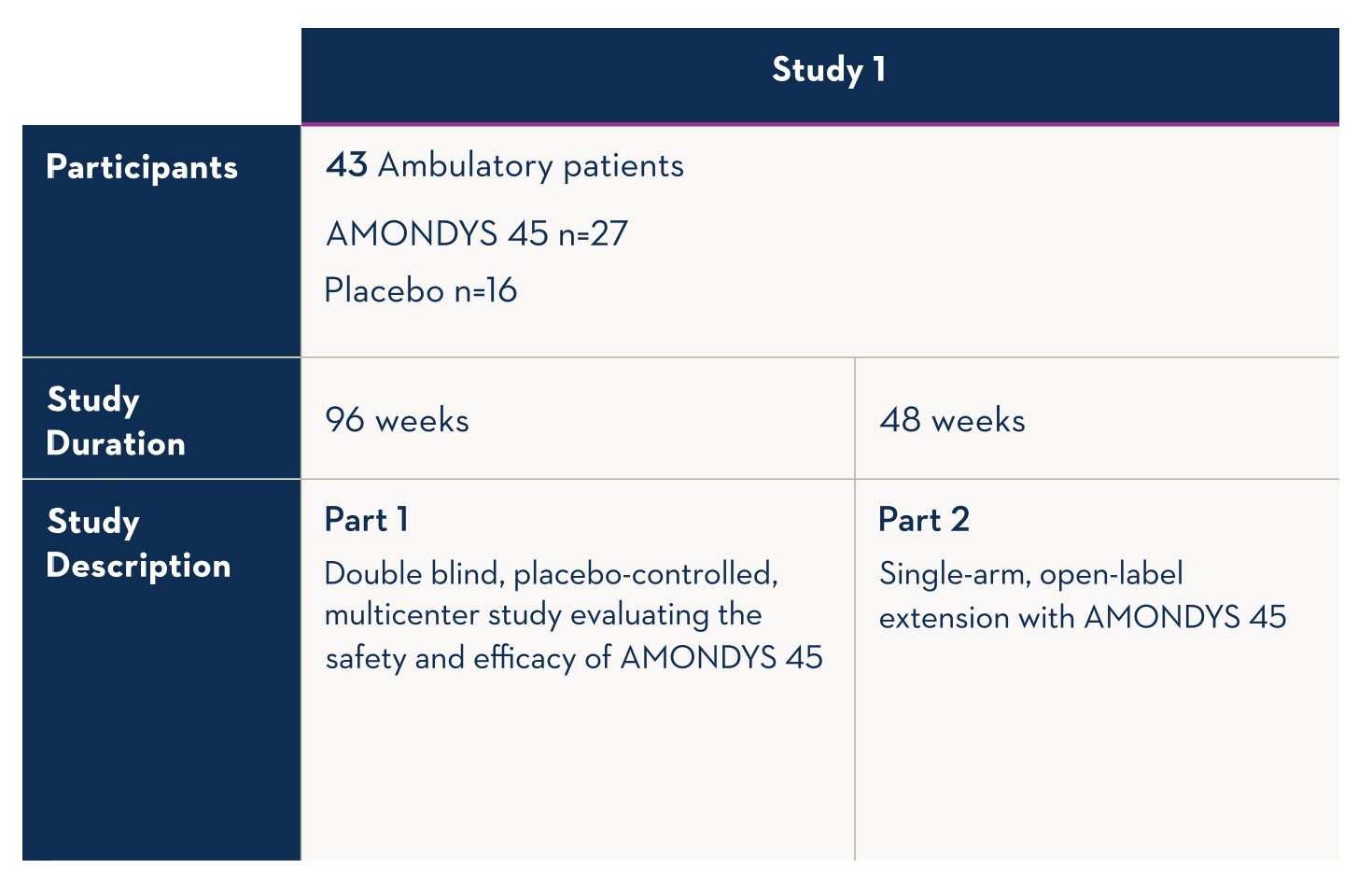
The ongoing Study 1 looks at whether exon 45 skipping occurred on the dystrophin gene of boys treated with AMONDYS 45.
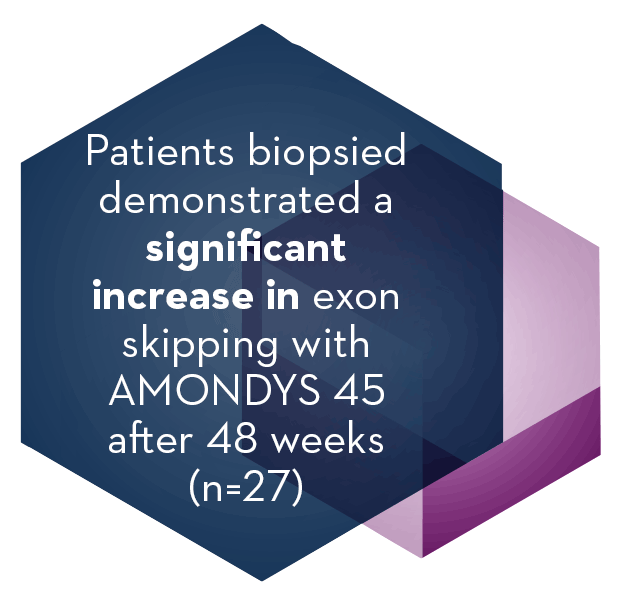
In the interim analysis of muscle biopsy tissue obtained at baseline and at Week 48 from patients in Study 1, patients who received AMONDYS 45 (n=27) demonstrated a significant increase in skipping of exon 45 (P<0.001) compared to baseline.
Patients who received placebo (n=16) did not demonstrate a significant increase in exon 45 skipping (P=0.808).
The level of exon skipping was positively correlated with dystrophin protein expression.
y-axis represents 5% on a scale of 100%
*Healthy subjects = people without DMD.

SareptAssist is a support program designed to help patients seeking information on AMONDYS 45 (casimersen). Our dedicated team will provide information on:


Sign up to get news and information from Sarepta Therapeutics, including updates on Sarepta products and services.
* denotes a required field
AMONDYS 45 is used to treat patients with Duchenne muscular dystrophy (DMD) who have a confirmed mutation of the dystrophin gene that can be treated by skipping exon 45.
This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with AMONDYS 45. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
Contraindications: Do not receive AMONDYS 45 if you are allergic to casimersen or any of the ingredients in AMONDYS 45. Serious allergic reactions to casimersen have included anaphylaxis, which may include difficulty breathing, tightness in the chest, and angioedema which may include swelling of the mouth, face, lips, or tongue.
Hypersensitivity Reactions: Serious allergic reactions, including angioedema and anaphylaxis, have occurred in patients who were treated with AMONDYS 45. Patients should seek immediate medical care should they experience signs and symptoms of allergic reactions. Your doctor will institute appropriate medical treatment which may include slowing, interrupting, or discontinuing the AMONDYS 45 infusion. Your doctor will monitor you until the condition resolves.
Kidney Toxicity and Monitoring: Damage to the kidneys was seen in animals who received casimersen. Although damage to the kidneys was not seen in clinical studies with AMONDYS 45, potentially fatal kidney damage has occurred with other drugs that work in a similar way. Your doctor may recommend urine and blood testing before starting treatment followed by urine testing every month and a blood test every 3 months to monitor your kidneys.
Adverse Reactions: Side effects occurring in at least 20% of patients treated with AMONDYS 45 and at least 5% more frequently than in patients who received an inactive intravenous (IV) infusion were (AMONDYS 45, placebo): upper respiratory tract infection (65%, 55%), cough (33%, 26%), fever (33%, 23%), headache (32%, 19%), joint pain (21%, 10%), and pain in mouth and throat (21%, 7%).
Other side effects that occurred in at least 10% of patients treated with AMONDYS 45 and at least 5% more frequently than patients who received an inactive IV infusion were: ear pain, nausea, ear infection, pain after injury, and dizziness and light-headedness.
What do I do if I have side effects?
Ask your healthcare provider for advice about any side effects that concern you.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
The information provided here does not include all that is known about AMONDYS 45. To learn more, talk with your healthcare provider.
Before receiving this infusion, please see the full Prescribing Information for AMONDYS 45 (casimersen).
Contraindications: Do not receive AMONDYS 45 if you are allergic to casimersen or any of the ingredients in AMONDYS 45. Serious allergic reactions to casimersen have included anaphylaxis, which may include difficulty breathing, tightness in the chest, and angioedema which may include swelling of the mouth, face, lips, or tongue.
Hypersensitivity Reactions: Serious allergic reactions, including angioedema and anaphylaxis, have occurred in patients who were treated with AMONDYS 45. Patients should seek immediate medical care should they experience signs and symptoms of allergic reactions. Your doctor will institute appropriate medical treatment which may include slowing, interrupting, or discontinuing the AMONDYS 45 infusion. Your doctor will monitor you until the condition resolves.
Kidney Toxicity and Monitoring: Damage to the kidneys was seen in animals who received casimersen. Although damage to the kidneys was not seen in clinical studies with AMONDYS 45, potentially fatal kidney damage has occurred with other drugs that work in a similar way. Your doctor may recommend urine and blood testing before starting treatment followed by urine testing every month and a blood test every 3 months to monitor your kidneys.
Adverse Reactions: Side effects occurring in at least 20% of patients treated with AMONDYS 45 and at least 5% more frequently than in patients who received an inactive intravenous (IV) infusion were (AMONDYS 45, placebo): upper respiratory tract infection (65%, 55%), cough (33%, 26%), fever (33%, 23%), headache (32%, 19%), joint pain (21%, 10%), and pain in mouth and throat (21%, 7%).
AMONDYS 45 is used to treat patients with Duchenne muscular dystrophy (DMD) who have a confirmed mutation of the dystrophin gene that can be treated by skipping exon 45.
This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with AMONDYS 45. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
Contraindications: Do not receive AMONDYS 45 if you are allergic to casimersen or any of the ingredients in AMONDYS 45. Serious allergic reactions to casimersen have included anaphylaxis, which may include difficulty breathing, tightness in the chest, and angioedema which may include swelling of the mouth, face, lips, or tongue.
Hypersensitivity Reactions: Serious allergic reactions, including angioedema and anaphylaxis, have occurred in patients who were treated with AMONDYS 45. Patients should seek immediate medical care should they experience signs and symptoms of allergic reactions. Your doctor will institute appropriate medical treatment which may include slowing, interrupting, or discontinuing the AMONDYS 45 infusion. Your doctor will monitor you until the condition resolves.
Kidney Toxicity and Monitoring: Damage to the kidneys was seen in animals who received casimersen. Although damage to the kidneys was not seen in clinical studies with AMONDYS 45, potentially fatal kidney damage has occurred with other drugs that work in a similar way. Your doctor may recommend urine and blood testing before starting treatment followed by urine testing every month and a blood test every 3 months to monitor your kidneys.
Adverse Reactions: Side effects occurring in at least 20% of patients treated with AMONDYS 45 and at least 5% more frequently than in patients who received an inactive intravenous (IV) infusion were (AMONDYS 45, placebo): upper respiratory tract infection (65%, 55%), cough (33%, 26%), fever (33%, 23%), headache (32%, 19%), joint pain (21%, 10%), and pain in mouth and throat (21%, 7%).
Other side effects that occurred in at least 10% of patients treated with AMONDYS 45 and at least 5% more frequently than patients who received an inactive IV infusion were: ear pain, nausea, ear infection, pain after injury, and dizziness and light-headedness.
What do I do if I have side effects?
Ask your healthcare provider for advice about any side effects that concern you.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
The information provided here does not include all that is known about AMONDYS 45. To learn more, talk with your healthcare provider.
Before receiving this infusion, please see the full Prescribing Information for AMONDYS 45 (casimersen).