Side effects were evaluated in a clinical study.
As with any medication, it’s important to talk to your doctor about the possibility of side effects with AMONDYS 45. Here’s what you should know about side effects experienced by those receiving AMONDYS 45 in clinical studies.
Allergic reactions can happen.
Serious allergic reactions, including angioedema (swelling under the skin, which may include mouth, face, lips, or tongue) and anaphylaxis (a serious, potentially life-threatening allergic reaction which may include difficulty breathing and tightness in the chest), have occurred in patients who were treated with AMONDYS 45.
Seek immediate medical care if signs and symptoms of allergic reactions happen.
Kidney Monitoring for Safety
Damage to the kidneys was seen in animals who received casimersen. Although damage to the kidneys was not seen in clinical studies with AMONDYS 45, potentially fatal kidney damage has occurred with other drugs that work in a similar way. Your doctor may recommend urine and blood testing before starting treatment followed by urine testing every month and a blood test every 3 months to monitor your kidneys.
Common Side Effects
Side effects observed in at least 20% of treated patients and at least 5% more often than in an inactive IV infusion (placebo).
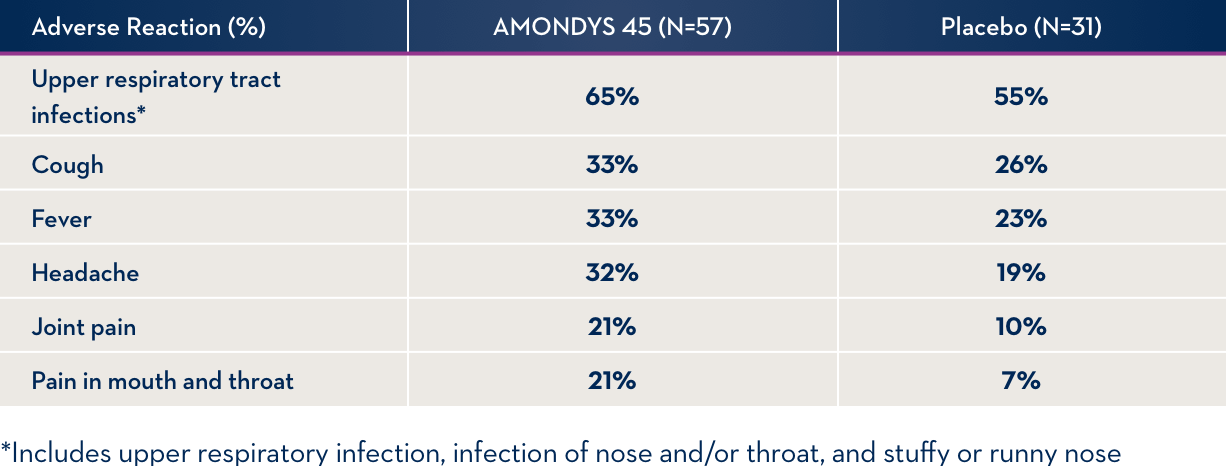
Other Side Effects
Other side effects that occurred in at least 10% of patients treated with AMONDYS 45, and that were reported at a rate at least 5% more frequently in the AMONDYS 45 group than in the placebo group, were:
• Ear pain
• Nausea
• Ear infection
• Pain after injury
• Dizziness and light-headedness

A phone call away
As with any medication, please talk to your doctor if you experience any side effects from AMONDYS 45. You are encouraged to report negative side effects of all prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Before receiving this infusion, please see the full Prescribing Information for AMONDYS 45 (casimersen).
Related FAQs
Individuals who are allergic to casimersen or any of the ingredients in AMONDYS 45 should not receive AMONDYS 45. Serious allergic reactions to casimersen have included anaphylaxis, which may include difficulty breathing, tightness in the chest, and angioedema, which may include swelling of the mouth, face, lips, or tongue.
The instructions you get from your doctor will explain when you should contact them. Always contact your doctor if:
- You notice any redness, tenderness, bruising, swelling, warmth, or drainage at or near the infusion site
- You experience swelling, tingling, or pain at or near the port infusion site or in the arm closest to the port
- You develop a fever
*Always refer to the manufacturer’s instruction for use (IFU) guide for more information on safety and precautions and ask your healthcare provider to review the relevant instruction for use of your port with you.
