
An exon-skipping therapy used to treat Duchenne.
AMONDYS 45 is used to treat patients with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the dystrophin gene that can be treated by skipping exon 45.
This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with AMONDYS 45.
Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
Beck’s Journey with AMONDYS 45
Embark on Beck’s journey with AMONDYS 45 as he and his family share their treatment goals and experiences, how AMONDYS 45 fits into their lives, and the vital role SareptAssist plays in their journey.
How does it work?
AMONDYS 45 is an exon-skipping therapy that helps the body make a shorter form of the dystrophin protein.a Find out more about AMONDYS 45 and the results from an ongoing clinical study.
aBoys treated with AMONDYS 45 showed varying levels of increased dystrophin production after 48 weeks. In an ongoing clinical study, 27 boys (median age 9 years) receiving AMONDYS 45 had an average dystrophin level of 1.74% of normal, compared to 0.76% in 16 boys who received a placebo infusion.
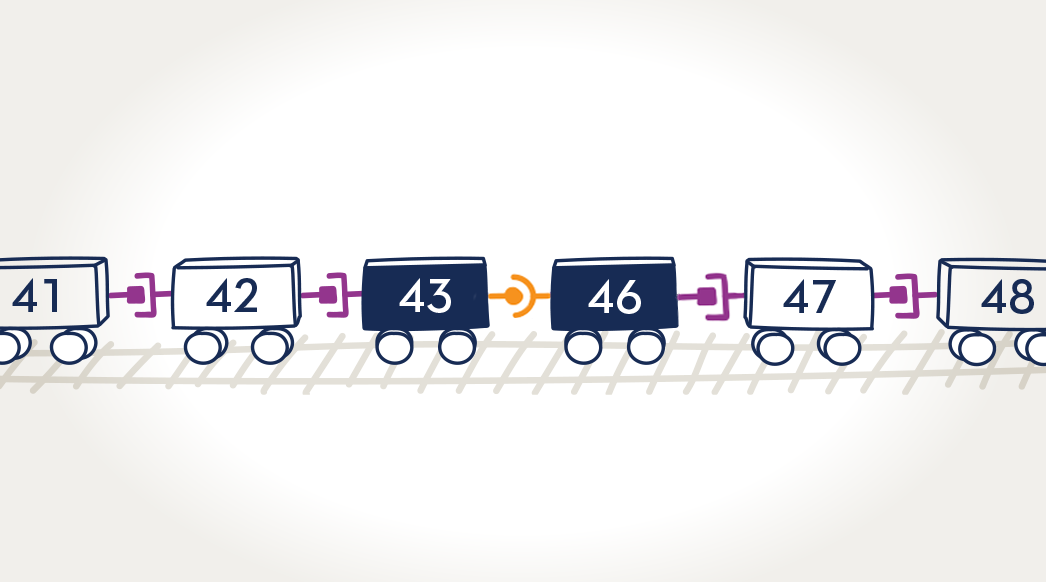
A lack of dystrophin protein
Exon-skipping technologies strive to address the underlying issue with Duchenne—a lack of the protein dystrophin. Many people with Duchenne have a genetic mutation in which one or more exons in the dystrophin gene are missing. This causes errors in the instructions for making dystrophin, leaving the body unable to produce the protein. Exon skipping tells the body to hide an exon so the whole section can be skipped over and the remaining exons can fit together. AMONDYS 45 is designed to skip over an exon. AMONDYS 45 helped boys with DMD amenable to skipping exon 45 to make a shorter form of dystrophin protein.a
aBoys treated with AMONDYS 45 showed varying levels of increased dystrophin production after 48 weeks. In an ongoing clinical study, 27 boys (median age 9 years) receiving AMONDYS 45 had an average dystrophin level of 1.74% of normal, compared to 0.76% in 16 boys who received a placebo infusion.
Informed decisions.
There are certain risks and side effects associated with AMONDYS 45. As with any medication, you should discuss risks and side effects with your child's doctor.

